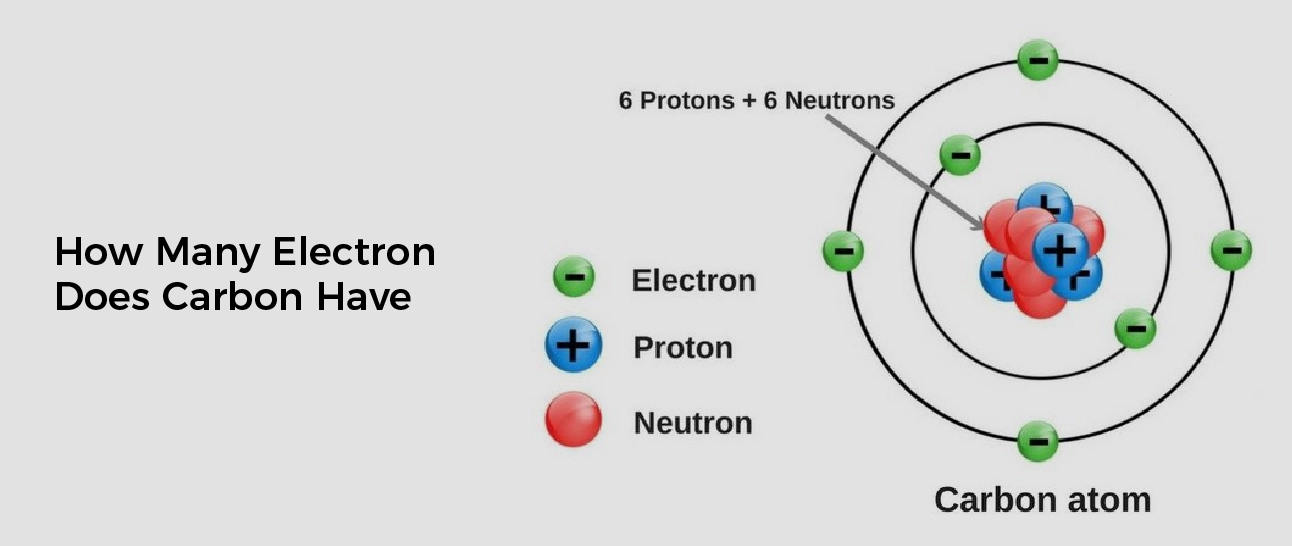
A neutral carbon atom has 6 electrons. In this article, we will study in detail the number of electrons carbon has.
Carbon
Carbon is a chemical element and has the chemical symbol "C" and atomic number 6. It is a non-metallic element that is essential to all known forms of life, as it is a key component of organic molecules such as proteins, DNA, and carbohydrates.
Carbon is unique in its ability to form long chains and complex structures, which allows it to participate in an almost infinite number of chemical reactions. It is also a major component of many minerals and fuels, including coal, oil, and natural gas.
Carbon has a variety of physical and chemical properties, including its ability to exist in multiple allotropes, such as diamond, graphite, and fullerenes, which have different properties and applications.
1. Number of Electrons
In Carbon Electrons in an atom spin around the center, also known as the nucleus. The electrons prefer to be in different shells / orbitals.
Shell number one can only hold two electrons, shell number two can hold eight, and shell number three can hold up to eight electrons for the first eighteen elements. Shell three can hold more than eight electrons, as you come across elements with more than eighteen electrons.
The next electron must move to the next shell when one shell is full. A neutral carbon atom has 6 electrons. The atomic number of carbon is 6, which means that a neutral carbon atom has 6 protons and 6 electrons.
In a stable carbon atom, the number of protons (positive charges) is equal to the number of electrons (negative charges), which results in a neutral charge for the atom. However, in some chemical reactions, carbon can lose or gain electrons, which can result in a positively or negatively charged ion.

2. Electronic Configuration of Carbon
The electronic configuration of carbon is 1s² 2s² 2p², meaning it has a total of 6 electrons arranged in different atomic orbitals.
The first energy level (n = 1) holds 2 electrons in the 1s subshell, while the second energy level (n = 2) holds the remaining 4 electrons—2 in the 2s subshell and 2 in the 2p subshell. This arrangement plays a key role in carbon’s ability to form diverse chemical bonds.
In the case of carbon, the first two electrons occupy the 1s subshell, while the next two occupy the 2s subshell. The remaining two electrons are in the 2p subshell, with one electron each in two of the three available 2p orbitals (2px and 2py).
The electronic configuration can also be written as [He] 2s2 2p2, which indicates that carbon's electron configuration is the same as the noble gas helium's configuration in its filled 1s orbital.
Key Takeaways
Carbon has the chemical symbol C.
The atomic number of Carbon is 6.
The electronic configuration of carbon is 2, 4.
Conclusion
In conclusion, creating engaging and thoughtful content is crucial for maintaining an active and appealing blog. Whether you are sharing personal experiences, offering advice, or discussing industry trends, make sure your writing is clear and resonates with your audience.
Consistency in posting, along with interactive elements like comments and social media sharing, can enhance reader engagement. Remember to stay true to your voice and mission, and continuously seek to improve your skills and understanding of your audience's needs. Happy blogging!
FAQs
1. How many electrons does a carbon atom have?
A neutral carbon atom has 6 electrons.
2. Why does carbon have 6 electrons?
Carbon has 6 electrons because its atomic number is 6, which means it has 6 protons in its nucleus. In a neutral atom, the number of electrons is equal to the number of protons.
3. How are carbon's electrons arranged?
Carbon's electrons are arranged in different atomic orbitals, specifically in the 1s, 2s, and 2p orbitals. The 1s orbital contains 2 electrons, while the 2s and 2p orbitals can hold up to 4 electrons.
4. Why is the number of electrons important for carbon?
The number of electrons in an atom determines its chemical behavior and its ability to bond with other atoms. Carbon has 4 valence electrons, which allows it to form strong covalent bonds with other atoms, including other carbon atoms, hydrogen, oxygen, and many other elements.
5. How does the number of electrons in carbon affect its properties?
Carbon's ability to form strong covalent bonds and to bond with a wide variety of elements is a key factor in its many important properties, including its ability to form complex molecules, its role in biological systems, and its use in a wide range of industrial applications.




Comments