The atomic number of an element is the number of protons present in one atom. In this article, we will study about atomic number and how can it be determined.
Atomic Number- Definition
The atomic number of an element is the number of protons present in the nucleus of its atom. It is a unique identity of every element and also determines its chemical properties.
In a neutral atom, the atomic number is equal to the number of electrons. This is why the atomic number is the basis for arranging elements in the Periodic Table.
Atoms of the same element may have the same atomic number but different numbers of neutrons. These variants are called isotopes. Isotopes have the same chemical properties but different atomic masses.
1. Notation of Atomic Number
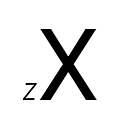
The notation for an atomic number is Z. It is usually represented as a subscript to the left of the element symbol.
In the periodic table, the atomic number is typically listed above the symbol for the element. For example, the symbol for Nitrogen is "N" and the atomic number is listed as "7".
Note
The atomic number should not to be confused with the atomic mass or atomic weight.
2. Can an Atomic Number of an Element Change?
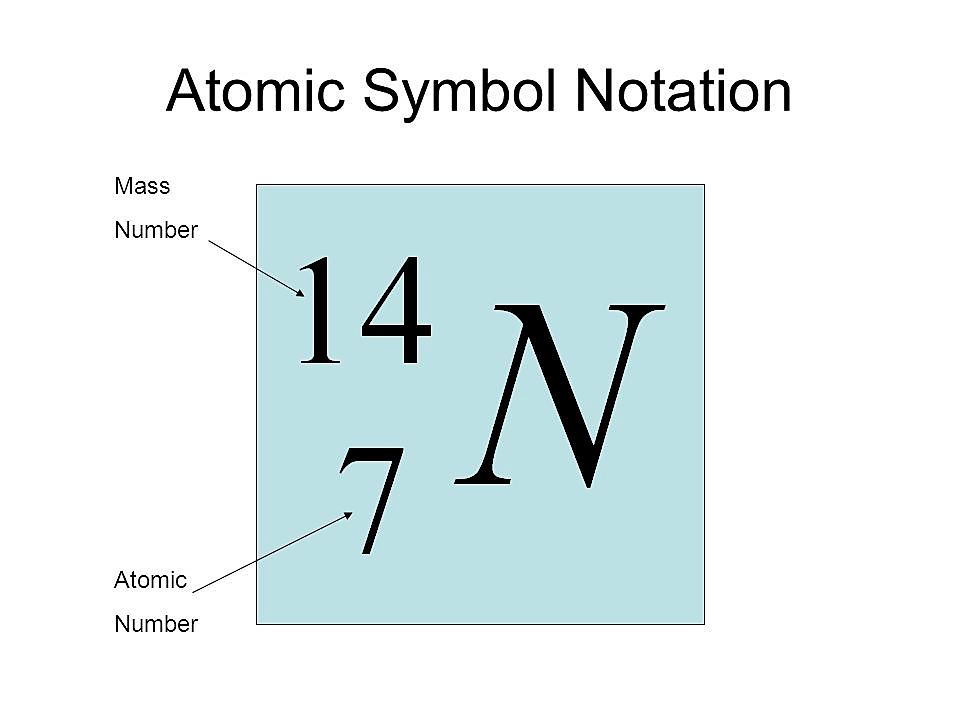
The atomic number of an element can also be defined as the number of electrons present in the neutral atom of that element.
Atomic number of an element = Number of electrons Only a neutral atom contains the same number of electrons and protons. Therefore, the atomic number of an element can also be equal to the number of electrons in the neutral atom and not in the ion.
An ion is formed by removing electrons from a neutral atom or adding electrons to a neutral atom, and thus contains either fewer or more electrons than protons. Only the electron of an atom participates in chemical reactions, and the protons do not participate.
So, while the number of electrons may vary during a chemical reaction, only the number of protons remains constant.
As a result, the atomic number does not change during a chemical reaction and remains constant.
3. Importance of Atomic Number
The atomic number of an element is an important concept in chemistry and physics because it provides a means of uniquely identifying and classifying elements.
The following are some of the key importance of the atomic number:
Determines the chemical properties of an element: The atomic number of an element determines the number of electrons in the neutral atom of that element, which in turn determines the chemical properties and reactivity of the element.
Arranging elements in the periodic table: The atomic number of an element is used to arrange elements in the periodic table. The elements are arranged in order of increasing atomic number, which allows for the prediction of the chemical and physical properties of elements based on their position in the periodic table.
Determine the number of isotopes: The atomic number of an element determines the number of isotopes that can exist for that element. Isotopes are atoms of the same element that have the same number of protons, but different numbers of neutrons, resulting in different atomic masses.
Used in nuclear physics: The atomic number is also important in the study of nuclear physics, as it determines the number of protons in the nucleus of an atom and influences the stability and decay of the nucleus.
Identification of elements in compounds: The atomic number is used to identify the elements present in a compound and to calculate the chemical formula for the compound.
4. How to find Atomic Number?
The atomic number is the total number of protons present in the nucleus of an atom of that element. It is a unique property of an element and determines the chemical properties of the element.
To find the atomic number of an element, you can consult a periodic table of elements. The periodic table is arranged in an increasing order of atomic number, so you can simply look up the element you are interested in and find its atomic number listed next to its symbol.
Alternatively, the atomic number can also be determined on the basis of the number of electrons in the neutral atom of that element. The number of electrons in a neutral atom is equal/ same to the number of protons, which is the atomic number.
5. Atomic Number of s and p block elements
Below given is the list of Atomic number of s and p block elements:
Element | Symbol | No. of protons | No. of electrons | Atomic Number | Block |
Hydrogen | H | 1 | 1 | 1 | S |
Helium | He | 2 | 2 | 2 | S |
Lithium | Li | 3 | 3 | 3 | S |
Beryllium | Be | 4 | 4 | 4 | S |
Boron | B | 5 | 5 | 5 | P |
Carbon | C | 6 | 6 | 6 | P |
Nitrogen | N | 7 | 7 | 7 | P |
Oxygen | O | 8 | 8 | 8 | P |
Fluorine | F | 9 | 9 | 9 | P |
Neon | Ne | 10 | 10 | 10 | P |
Sodium | Na | 11 | 11 | 11 | S |
Magnesium | Mg | 12 | 12 | 12 | S |
Aluminium | Al | 13 | 13 | 13 | P |
Silicon | Si | 14 | 14 | 14 | P |
Phosphorus | P | 15 | 15 | 15 | P |
Sulfur | S | 16 | 16 | 16 | P |
Chlorine | Cl | 17 | 17 | 17 | P |
Argon | Ar | 18 | 18 | 18 | P |
Potassium | K | 19 | 19 | 19 | S |
Calcium | Ca | 20 | 20 | 20 | S |
Gallium | Ga | 31 | 31 | 31 | P |
Germanium | Ge | 32 | 32 | 32 | P |
Arsenic | As | 33 | 33 | 33 | P |
Selenium | Se | 34 | 34 | 34 | P |
Bromine | Br | 35 | 35 | 35 | P |
Krypton | Kr | 36 | 36 | 36 | P |
Rubidium | Rb | 37 | 37 | 37 | S |
Strontium | Sr | 38 | 38 | 38 | S |
Indium | In | 49 | 49 | 49 | P |
Tin | Sn | 50 | 50 | 50 | P |
Antimony | Sb | 51 | 51 | 51 | P |
Tellurium | Te | 52 | 52 | 52 | P |
Iodine | I | 53 | 53 | 53 | P |
Xenon | Xe | 54 | 54 | 54 | P |
Cesium | Cs | 55 | 55 | 55 | S |
Barium | Ba | 56 | 56 | 56 | S |
Thallium | Tl | 81 | 81 | 81 | P |
Lead | Pb | 82 | 82 | 82 | P |
Bismuth | Bi | 83 | 83 | 83 | P |
Polonium | Po | 84 | 84 | 84 | P |
Astatine | At | 85 | 85 | 85 | P |
Radon | Rn | 86 | 86 | 86 | P |
Francium | Fr | 87 | 87 | 87 | S |
Radium | Ra | 88 | 88 | 88 | S |
Nihonium | Nh | 113 | 113 | 113 | P |
Flerovium | Fl | 114 | 114 | 114 | P |
Moscovium | Mc | 115 | 115 | 115 | P |
Livermorium | Lv | 116 | 116 | 116 | P |
Tennessine | Ts | 117 | 117 | 117 | P |
Oganesson | Og | 118 | 118 | 118 | P |
Key Takeaways
The atomic number of an element is the number of protons present in one atom.
In a neutral atom, atomic number can also be the number of electrons of an atom.
An atomic number is denoted by the letter Z which is written at the subscript on the left side of the chemical symbol.
The atomic number is an essential concept in the study of chemistry and physics, providing a means of identifying and classifying elements and determining their chemical and physical properties.
Conclusion
In conclusion, effective blogging requires a blend of creativity, consistency, and engagement with your audience. By understanding your niche, crafting valuable content, and promoting it through the right channels, you can build a successful blog that resonates with readers.
Remember, the journey of blogging is a continuous learning experience, so keep exploring new ideas and strategies to enhance your content and grow your community. Happy blogging!
FAQs
1. How to determine the atomic number of an element?
The atomic number of an element is the total number of protons present in the nucleus of an atom of that element. This number is a fundamental property of an element and cannot be changed through chemical reactions.
2. Can the atomic number change?
No, the atomic number of an element cannot change. The atomic number is a unique property of an element and determines the chemical and physical properties of the element.
3. What would happen if an atom gains or loses protons?
If an atom gains or loses protons, it will become a different element. This process is known as nuclear decay and is a fundamental aspect of nuclear physics. The addition or removal of a proton changes the atomic number of the element and its chemical and physical properties.
4. How does the atomic number relate to isotopes?
Isotopes are atoms of the same element having the same number of protons, but differs in the numbers of neutrons. The atomic number of a particular element is the same as that of the number of protons present in the nucleus of an atom, and determines the number of isotopes that can exist for that element.
5. How does the atomic number relate to the electron configuration of an element?
The electron configuration of an element is determined by the number of electrons in the neutral atom of that element, which is equal to the atomic number of the element. The electron configuration of an element determines its chemical and physical properties.




Comments