Valence electrons can be defined as electrons that are present in the outermost electrons of an atom.
They are responsible for the chemical behavior of an element and are involved in the formation of chemical bonds. The valence electrons of an atom determine its chemical properties and reactivity.
In this article, we will study in detail about Valence electrons. In order to understand what valence electrons are, let us first understand valency.
- Valency
- Conclusion
- FAQs
- 1. What are valence electrons?
- 2. How do valence electrons affect the reactivity of an atom?
- 3. How do valence electrons determine the chemical properties of an element?
- 4. How can you determine the number of valence electrons in an atom?
- 5. What is the difference between valence electrons and core electrons?
Valency
Valency: The Combining Capacity of Elements
Valency is the combining capacity of an element, meaning the number of chemical bonds an atom can form with other atoms. It depends on the valence electrons—the electrons present in the outermost shell of an atom.
Atoms with 1 or 2 valence electrons have a valency of 1 or 2. For example, hydrogen (H) has a valency of 1, while oxygen (O) has a valency of 2.
Atoms with 3 or more valence electrons usually have a higher valency and can form multiple bonds. For example, nitrogen (N) has a valency of 3, and carbon (C) has a valency of 4.
In simple words, valency tells us how many bonds an atom can make in order to achieve a stable electronic configuration.
1. Valence Electrons
The valence electrons are the electrons that are present in the outermost shell of an atom and are responsible for the chemical behavior of an element.
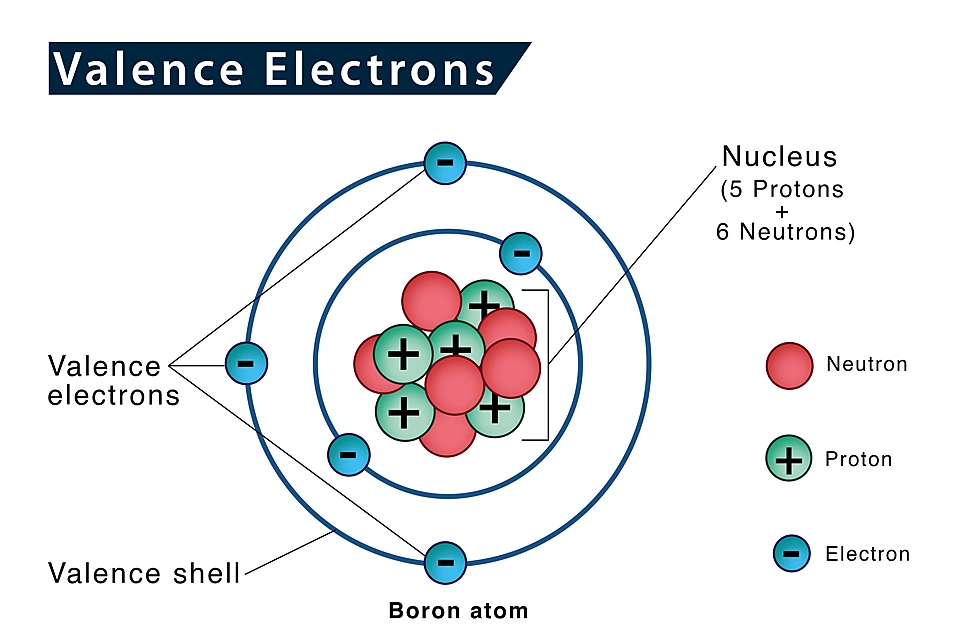
The number of valence electrons of some groups of the periodic table is listed below.
The group in the Periodic Table | Number of Valence Electrons |
Group 1 (I A) | 1 |
Group 2 (II A) | 2 |
Group 13 (III A) | 3 |
Group 14 (IV A) | 4 |
Group 15 (V A) | 5 |
Group 16 (VI A) | 6 |
Group 17 (VII A) | 7 |
Group 18 (VIII A or 0) | 8 |
2. Relationship between Valency and Valence
Electrons Valency explains how atoms form bonds. Valence electrons, on the other hand, are associated with elemental properties. Valency is simply a concept or idea that does not involve electron transmission.
In contrast, valence electrons are involved in the transmission of electrons during the formation of bonds. Any chemical element can have both valency and valence electrons.
It is only the valence electrons (outermost electrons) that participate in chemical bonding, and the number of valence electrons in an element's atom determines its valency. The number of valence electrons determines the valency in its atom or the number of electrons required to complete eight electrons in the valence shell.
For example, sodium has one valence electron and thus a valency of one. As a result, sodium's valency equals the number of valence electrons in its atom. In general, the valency of a metal element is the number of valence electrons in its atom.
Like electrons in the inner shell, a valence electron can receive or emit photon energy. When an electron gains enough energy to migrate (jump) to an outer shell, this is referred to as atomic excitation.
Ionization occurs when an electron breaks from the valence shell of its associated atom, resulting in the formation of a positive ion. When an electron loses energy (and thus emits a photon), it may migrate to a partially occupied inner shell.
3. Characteristics of Valence Electrons
Valence electrons have certain characteristics that make them unique from other electrons in an atom. These include:
Location: Valence electrons are located in the outermost energy level or valence shell of an atom.
Number: The number of valence electrons in an atom is determined by its position in the periodic table and is related to its valency.
Reactivity: Valence electrons are responsible for the chemical behavior of an atom and are involved in the formation of chemical bonds. The valence electrons in an atom determine its reactivity and chemical properties.
Involvement in chemical reactions: Valence electrons are the ones that are involved in the chemical reactions, they are the ones that form chemical bonds with other atoms.
Electron shielding: Valence electrons are shielded by the inner-shell electrons from the full effect of the atomic nucleus, this effect causes the valence electrons to be more easily influenced by other atoms and molecules.
Electron configuration: The valence electrons of an atom are the ones that occupy the highest energy level of the atom, this is why valence electrons are also known as outer-shell electrons.
4. Significance of Valence Electrons
Valence electrons are significant because they determine how an atom will interact with other atoms.
The reactivity and chemical properties of an element can be determined by the number of valence electrons it has. Atoms with similar numbers of valence electrons tend to have similar chemical properties and reactivity.
For example, elements in the same group or column of the periodic table typically have the same number of valence electrons and therefore have similar chemical properties. Additionally, the number of valence electrons determines the type of chemical bond that an atom can form.
For example, atoms with one or two valence electrons tend to form ionic bonds, while atoms with three or more valence electrons tend to form covalent bonds.
5. How to Determine the Valence Electrons of an Atom?
There are a few ways to determine the valence electrons of an atom:
Using the Periodic Table: The number of valence electrons in an atom can be determined by looking at its position in the periodic table. Elements in the same group or column of the periodic table typically have the same number of valence electrons.
Electron Configuration: The valence electrons of an atom can be determined by looking at its electron configuration. The valence electrons are the outermost electrons in an atom and are the ones in the highest energy level or highest principal quantum number.
Lewis Dot Structures: A Lewis Dot Structure is a way to show the valence electrons of an atom by placing dots around the symbol of the element. The number of dots equals the number of valence electrons of the element.
The Aufbau Principle: Using the Aufbau principle, you can determine the valence electrons of an atom by filling the electron in the atomic orbitals according to the increased energy levels. The valence electrons are the ones that occupy the highest energy level of the atom.
The octet rule: The octet rule states that atoms tend to have 8 electrons in their valence shell in order to achieve a stable electron configuration. This rule can be used to predict the number of valence electrons for elements in Groups 1A, 2A, and 3A-8A.
Valence
Electrons of s and p block elements Below given is the list of number of electrons present in the outermost shell or valence electrons of s and p block elements:
Element | Symbol | Atomic Number | Valence electrons | Block |
Hydrogen | H | 1 | 1 | S block |
Helium | He | 2 | 2 | S block |
Lithium | Li | 3 | 1 | S block |
Beryllium | Be | 4 | 2 | S block |
Boron | B | 5 | 3 | P block |
Carbon | C | 6 | 4 | P block |
Nitrogen | N | 7 | 5 | P block |
Oxygen | O | 8 | 6 | P block |
Fluorine | F | 9 | 7 | P block |
Neon | Ne | 10 | 8 | P block |
Sodium | Na | 11 | 1 | S block |
Magnesium | Mg | 12 | 2 | S block |
Aluminium | Al | 13 | 3 | P block |
Silicon | Si | 14 | 4 | P block |
Phosphorus | P | 15 | 5 | P block |
Sulfur | S | 16 | 6 | P block |
Chlorine | Cl | 17 | 7 | P block |
Argon | Ar | 18 | 8 | P block |
Potassium | K | 19 | 1 | S block |
Calcium | Ca | 20 | 2 | S block |
Gallium | Ga | 31 | 3 | P block |
Germanium | Ge | 32 | 4 | P block |
Arsenic | As | 33 | 5 | P block |
Selenium | Se | 34 | 6 | P block |
Bromine | Br | 35 | 7 | P block |
Krypton | Kr | 36 | 8 | P block |
Rubidium | Rb | 37 | 1 | S block |
Strontium | Sr | 38 | 2 | S block |
Indium | In | 49 | 3 | P block |
Tin | Sn | 50 | 4 | P block |
Antimony | Sb | 51 | 5 | P block |
Tellurium | Te | 52 | 6 | P block |
Iodine | I | 53 | 7 | P block |
Xenon | Xe | 54 | 8 | P block |
Cesium | Cs | 55 | 1 | S block |
Barium | Ba | 56 | 2 | S block |
Thallium | Tl | 81 | 3 | P block |
Lead | Pb | 82 | 4 | P block |
Bismuth | Bi | 83 | 5 | P block |
Polonium | Po | 84 | 6 | P block |
Astatine | At | 85 | 7 | P block |
Radon | Rn | 86 | 8 | P block |
Francium | Fr | 87 | 1 | S block |
Radium | Ra | 88 | 2 | S block |
Nihonium | Nh | 113 | 3 | P block |
Flerovium | Fl | 114 | 4 | P block |
Moscovium | Mc | 115 | 5 | P block |
Livermorium | Lv | 116 | 6 | P block |
Tennessine | Ts | 117 | 7 | P block |
Oganesson | Og | 118 | 8 | P block |
Key Takeaways
Valence electrons are the electrons present in the outermost shell of an atom.
Valency is the combining capacity of an element.
Valence electrons determine the valency of an element.
For metals, the valency of a metal is the number of valence electrons.
For non-metals, the valency of a non-metal is 8 – number of valence electrons.
Conclusion
In conclusion, creating engaging and meaningful content is essential for connecting with your audience on your Hyvor Blog. Whether you're sharing personal experiences, expert insights, or valuable information, always strive to provide value and foster a sense of community.
Remember to keep your writing authentic, use visuals to enhance your posts, and engage with your readers through comments and social media. By doing so, you'll not only strengthen your blog but also build lasting relationships with your audience.
Happy blogging!
FAQs
1. What are valence electrons?
Valence electrons are the outermost electrons of an atom. They are responsible for the chemical behavior of an element and are involved in the formation of chemical bonds. The number of valence electrons in an atom determines its chemical properties and reactivity.
2. How do valence electrons affect the reactivity of an atom?
Valence electrons determine the chemical properties and reactivity of an atom. Atoms with similar numbers of valence electrons tend to have similar chemical properties and reactivity. Additionally, the number of valence electrons determines the type of chemical bond that an atom can form.
3. How do valence electrons determine the chemical properties of an element?
The chemical properties of an element are determined by the number of valence electrons it has. Atoms with similar numbers of valence electrons tend to have similar chemical properties and reactivity. Elements in the same group or column of the periodic table typically have the same number of valence electrons and therefore have similar chemical properties.
4. How can you determine the number of valence electrons in an atom?
The number of valence electrons in an atom can be determined by looking at its position in the periodic table, its electron configuration, its Lewis dot structure, or by using the Aufbau principle or the octet rule.
5. What is the difference between valence electrons and core electrons?
Valence electrons are the outermost electrons of an atom and are responsible for the chemical behavior of an element. Core electrons, on the other hand, are the inner electrons of an atom and are not involved in chemical reactions.




Comments